Chemical Reactions of Haloalkanes
Chemical Reactions of Haloalkanes: Overview
This topic covers concepts, such as, Chemical Reactions of Haloalkanes, Nucleophilic Substitution Reactions of Alkyl Halides, Properties of Gem Dihalides & Reactions of Vicinal Dihalides etc.
Important Questions on Chemical Reactions of Haloalkanes
and reactions differ from each other because of
Identify A, B, C and D in the following process :
(i)
(ii)
What is the order of reactivity of following compounds towards substitutions reaction.
(i) 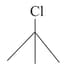
(ii) 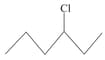
(iii) 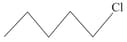
What is the order of reactivity of following compounds towards substitution reaction.
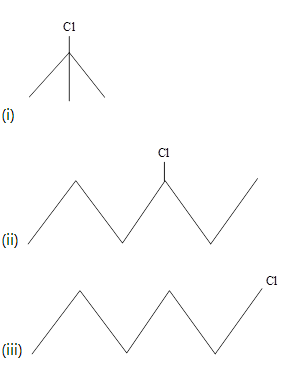
Which one of the following two substances undergoes reaction faster?
(i) 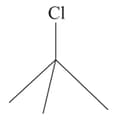 or (ii)
or (ii) 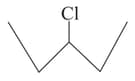
(CH3)3CMgCl on reaction with D2O produces –
An SN2 reaction at an asymmetric carbon of a compound always gives:
A solution of (+) -2-chloro-2-phenylethane in toluene racemises slowly in the presence of small amount of , due to the formation of –
A solution of (+) -2-chloro-1-phenylethane in toluene racemises slowly in the presence of small amount of , due to the formation of:
on treatment with produces:
1–chlorobutane on reaction with alcoholic potash gives –
n–Propyl bromide on treatment with ethanolic potassium hydroxide produces –
n–propyl bromide on treatment with ethanolic potassium hydroxide produces
The major product of the following reaction is
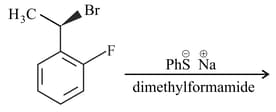
The product of following reaction is –
The reaction of -methyloct--ene with in the presence of gives two isomeric bromides in a ratio, with a combined yield of . Of these, the entire amount of the primary alkyl bromide was reacted with an appropriate amount of diethylamine followed by treatment with aq. to give a non-ionic product in Yield. The mass (in ) of obtained is
[Use molar mass (in ): ]
Which of the following is/are the property(ies) of ?
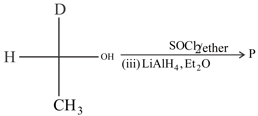
What is the configuration of product ?
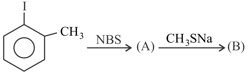
Product (B) is:
In the reaction,
The product C is.
